A paper on bioinspired synthesis of natural products isolated from Ericaceae plants has been published in J. Nat. Prod.!
- Kobayashi Shoji
- Dec 19, 2025
- 2 min read
Time flies, and there are only about two weeks left until end of 2025.
The lab is busy every day preparing master's and graduation theses and academic presentations.
Now, our paper has been published in the Journal of Natural Products!
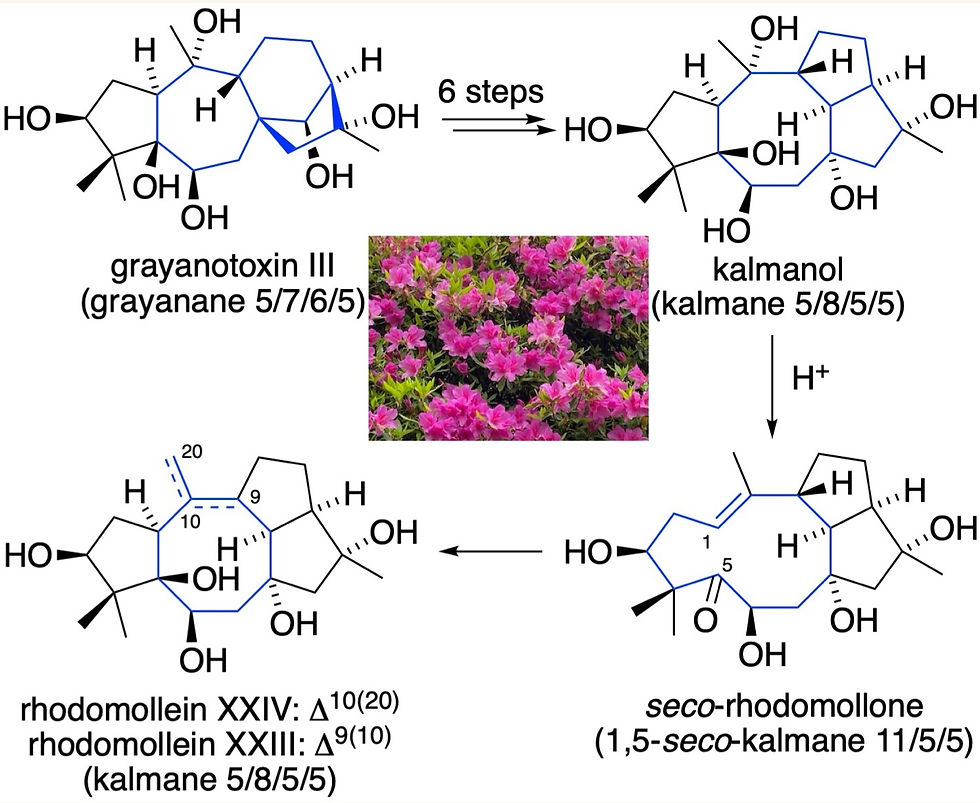
Bioinspired Chemical Transformations of Grayanotoxin III to Kalmanol, seco -Rhodomollone, and Rhodomolleins XXIII and XXIV
The original paper is here.
Ericaceae plants contain specific molecules called "grayanoids," which are classified as 20-carbon diterpenoids and are responsible for plant toxicity.
The molecular structures of grayanoids are extremely complex, consisting mainly of five- to eight-membered carbon rings, making a highly congested architecture.
To date, several hundred compounds have been discovered from Ericaceae plants.
We have successfully converted grayanotoxin, the most representative grayanoid isolated from the leaves of Leucothoe grayana Max., into other subtypes of grayanoids with different carbon frameworks, the so-called "kalmanol" and "rhodomollein".
Both kalmanol and rhodomollein are natural products isolated from plants of the Ericaceae family, with the former isolated from Kalmia angustifolia L. and the latter from Rhododendron molle.
This result chemically proves the hypothesis that these natural products, which have different skeletons, are produced from the same origin within plants, and is extremely useful in elucidating the whole picture of the grayanoid biosynthetic pathway (the process by which natural products are produced in living organisms).
The students involved in this project were Taiga Tsuruyama, who completed his master's degree in March this year, and Hinata Togo, a second-year master's student.
Using precious samples extracted from natural plants, they achieved their goal through painstaking experiments.
They must have been careful when conducting experiments using limited amounts of sample, but I believe that the experience of completing the project will be a great asset for them in the future.
Thank you for their efforts and congratulations on the publication!
However, in fact, we faced a serious problem towards the end of this research.
Based on past literature and preliminary experiments, we had assumed the reaction would proceed without any problems, but unexpectedly, it hardly progressed at all.
Viewing the results that a slight difference in molecular structure had significantly affected on the reactivity, I realized the difficulty of proving a hypothesis, and natural providence that produce specific molecules in living organisms.
Finally, I would like to thank Professor Tadamasa Terai for providing me with the valuable natural products.
Some days ago, a graduate of Terai's laboratory unexpectedly popped in my lab.
He said "I remember our lab continuously extracting natural products all year round."
Our research is based on the hard work of Professor Terai and his students at the time, and I would like to express my sincere gratitude.
We are very pleased to be able to publish our research results at the end of 2025.
Merry Christmas to everyone, and a slightly early Happy New Year!



Comments